Abstract
Vascular damage presents in many forms and varying geometries. Nevertheless, the platelet response to endothelial damage to the blood vessel wall, be it through a prick or a full puncture wound, is thought to be staged by a qualitatively similar temporal variance in signaling agonists. For example, endothelial damage in the microvasculature is thought to be initially dominated by thrombin and later by platelet released ADP and thromboxane. The same temporal sequence in signaling has been proposed to exist in a profusely bleeding puncture wound 1. If so, platelet morphology, a gold standard of platelet activation state, could provide a strong readout of temporally distinct signaling effects. Platelet morphology has long been considered to be a reliable indicator of a strong agonist such as thrombin acting through PAR receptors that produces a rounded, pseudopod extending, degranulated, highly adhesive platelet versus weaker agonists such as ADP or thromboxane acting through P2Y 12 receptors to produce a less adhesive, somewhat rounded platelet. A testable prediction of existing hemostasis models is that temporal staging of signaling leads to temporal differences in platelet morphology within the forming/remodeling thrombus. Such hypothesized temporal differences in signaling are clinically significant as they form the basis for hypothesizing phenotypically distinct outcomes for direct acting anti-coagulants (DOACs) affecting thrombin versus anti-platelet drugs affecting P2Y 12, ADP receptors.
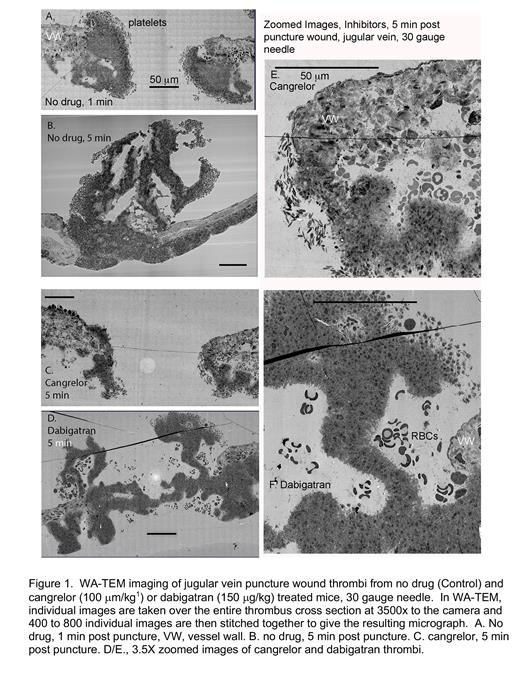
Advances in imaging, e.g., wide area transmission electron microscopy (WA-TEM), make possible the local determination of platelet activation state with high precision 2. Taking a mouse jugular vein puncture wound model 1,2, we found that all morphologically recognized platelet activation states were present early, 1 min post puncture, with loosely bound discoid shaped platelets being the most peripherally located. For bleeding, early-stage puncture wound, these loosely adherent, low activation state platelets were located on both intravascular and extravascular thrombus aggregates. Once the puncture wound is closed, loosely adherent platelets were only found on the intravascular surfaces of the thrombus. We propose that this result is most consistent with a platelet conversion model in which new loosely adherent platelets rapidly convert to tightly packed platelets. As the thrombus remodels, 5 and 20 min post-puncture, the thrombus continued to accumulate platelets both intravascularly and extravascularly. Peripheral, discoid shaped platelets provided a source for intravascular thrombus growth. However, any subsequent extravascular thrombus growth must be due to platelet migration. Significantly, we found that cangrelor, a direct acting P2Y 12 inhibitor, stalled thrombus formation/remodeling at an early stage (Figure 1A,C,E see also ref 1,2). By WA-TEM, the accumulation of discoid-shaped, loosely adherent platelets appeared to be enhanced in a cangrelor treated 5 min thrombus (Figure 1E,F). We suggest that P2Y 12 receptors must act early in thrombus formation with the conversion of discoid to more activated platelets being most affected. In contrast, a 5-min post puncture dabigatran (DOAC) treated showed deformed architecture with inhibition of the accumulation of discoid shaped platelets/rounded loosely adherent platelets being most affected (Figure 1D,F, see also ref 2). Accumulation of degranulated platelets appeared to be lessened in both cangrelor and dabigatran treated thrombi. We propose that the simplest explanation of these results is that multiple signaling pathways act in parallel with select activation states being more dependent on one pathway than another. Clinically, our results suggest that P2Y 12 inhibitors can affect thrombus formation at early time points in addition to the late time points projected by current models.
1. Tomaiuolo M., Matzko C.N., Posentud-Fuentes I., Weisel J.W., Brass L.F. & Stalker T.J. Interrelationships between structure and function during the hemostatic response to injury. Proc Natl Acad Sci USA. 116. 2243-2252 (2019).
2. Rhee, Pokrovskaya I.D.,BallK., LingK., VedanapartiY., CohenJ., CruzD., ZhaoO.S., AronovaM.A., ZhangG., Kamykowski J.A., LeapmanR.D., & StorrieB. Venous puncture wound hemostasis results in a vaulted thrombus structured by locally nucleated platelet aggregates. Commun. Biol., accepted.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal